Meso compound definition : A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters (chiral centers) it is not chiral. A meso compound is superimposable on its mirror image, and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. A meso compound need not even have a chiral center.
For example, there are 3 isomers of tartaric acid (depicted below): There is a meso compound (the 2R,3S and 2S,3R isomers are equivalent) and the optically active pair of dextrotartaric acid (L-(R,R)-(+)-tartaric acid) and levotartaric acid (D-(S,S)-(-)-tartaric acid). In the meso compound an internal plane of symmetry exists, bisecting the molecule which is not present in the non-meso compounds. That is, on rotating the meso compound by 180° on a plane perpendicular to the screen, the same stereochemistry is obtained, again this is not seen in the non-meso tartaric acid. (see Fischer projection).

It is a requirement for two of the stereocenters in a meso compound to have at least two substituents in common (though having this characteristic does not necessarily mean that the compound is meso). For example, in 2,4-pentanediol, both the second and fourth carbons, which are stereocenters, have all four substituents in common.
Since a meso isomer has a superposable mirror image, a compound with a total of n stereocenters cannot have 2n stereoisomers if at least one of the stereoisomers is meso.
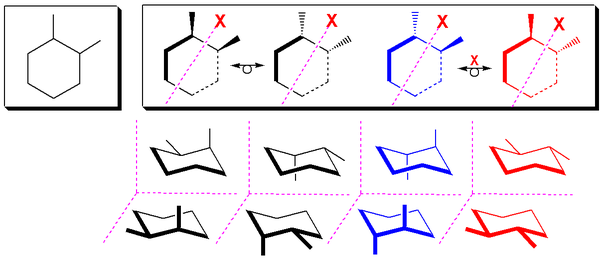
The two cis stereoisomers of 1,2-substituted cyclohexanes behave like meso compounds at room temperature in most cases. At room temperature, most 1,2-disubstituted cyclohexanes undergo rapid ring flipping (exceptions being rings with bulky substituents), and as a result, the two cis stereoisomers behave chemically identically with chiral reagents. At low temperatures, however, this is not the case, as the activation energy for the ring-flip cannot be overcome, and they therefore behave like enantiomers. A meso form is a type of stereoisomer which is optically inactive. In nearly all cases at room temperature, the two cis stereoisomers of 1,2-disubstituted cyclohexanes can be treated as chemically equivalent.[ Also noteworthy is the fact that when a cyclohexane undergoes a ring flip, the absolute configurations of the sterocenters do not change.
Reference wikipedia
Sponsored
For example, there are 3 isomers of tartaric acid (depicted below): There is a meso compound (the 2R,3S and 2S,3R isomers are equivalent) and the optically active pair of dextrotartaric acid (L-(R,R)-(+)-tartaric acid) and levotartaric acid (D-(S,S)-(-)-tartaric acid). In the meso compound an internal plane of symmetry exists, bisecting the molecule which is not present in the non-meso compounds. That is, on rotating the meso compound by 180° on a plane perpendicular to the screen, the same stereochemistry is obtained, again this is not seen in the non-meso tartaric acid. (see Fischer projection).

It is a requirement for two of the stereocenters in a meso compound to have at least two substituents in common (though having this characteristic does not necessarily mean that the compound is meso). For example, in 2,4-pentanediol, both the second and fourth carbons, which are stereocenters, have all four substituents in common.
Since a meso isomer has a superposable mirror image, a compound with a total of n stereocenters cannot have 2n stereoisomers if at least one of the stereoisomers is meso.
Cyclic meso compounds
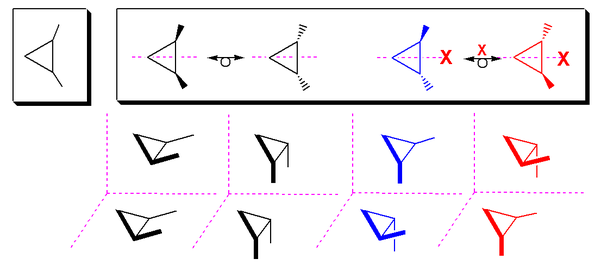
1,2-substituted cyclopropane has a meso cis-isomer (molecule has a mirror plane) and two trans-enantiomers:
The two cis stereoisomers of 1,2-substituted cyclohexanes behave like meso compounds at room temperature in most cases. At room temperature, most 1,2-disubstituted cyclohexanes undergo rapid ring flipping (exceptions being rings with bulky substituents), and as a result, the two cis stereoisomers behave chemically identically with chiral reagents. At low temperatures, however, this is not the case, as the activation energy for the ring-flip cannot be overcome, and they therefore behave like enantiomers. A meso form is a type of stereoisomer which is optically inactive. In nearly all cases at room temperature, the two cis stereoisomers of 1,2-disubstituted cyclohexanes can be treated as chemically equivalent.[ Also noteworthy is the fact that when a cyclohexane undergoes a ring flip, the absolute configurations of the sterocenters do not change.

Reference wikipedia