Stereogenic Elements and Stereoisomerism
Recognition of the three dimensional shape of molecules and the resulting symmetry implications is fundamental to an understanding of organic chemistry, especially stereoisomerism. The common occurrence of chiral centers, often asymmetric carbon units, in both natural and synthetic compounds has been described. In this section the treatment of chiral and achiral stereogenic elements will be extended to axes and
planes.
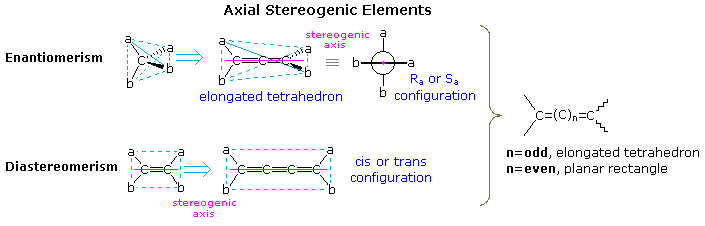
The following diagram illustrates the structural relationship of a disubstituted tetrahedral carbon (Ca
2b
2) to an allene (top row), and a disubstituted alkene (abC=Cab) to an analogous 1,2,3-cummulene. In each case the structure is elongated by the insertion of two additional carbons. As a result of this elongation, the symmetry
planes bisecting the a-C-a or b-C-b angles of the simple achiral tetrahedron are lost, and the allene is found to be chiral. Since no chiral center exists in this molecule, its chirality is due to the dissymmetric orientation of substituents about a
chiral axis (the axis defined by the three carbon atoms of the double bonds). No dramatic change of this kind is observed for the alkene elongation shown at the bottom. The cis / trans diastereoisomerism observed in achiral compounds of this kind is due to the same axial stereogenic element present in the alkene itself. A general rule relating the spatial orientation of terminal substituents in cumulenes of varying size to the number of sp-hybridized carbon atoms is shown on the right in the diagram.
To assign a stereochemical prefix, i.e.
Ra or
Sa (the subscript a refers to the axial chirality), to chiral configurations of this kind the structure must be viewed from one end of the stereogenic axis (it doesn't matter which). A Newman projection, like the one seen from the left shown here, is then used for the assignment. If the sequence order of substituents is a > b, then the two substituents nearest the viewer are assigned a ranking of 1 (a) and 2 (b), while the remote substituents are given rankings of 3 (a) and 4 (b). Applying the viewing rule then leads to a unique notation (
Ra in this case). This procedure may be used even when the A & B substituents on one sp
2 carbon are different from those on the other sp
2 carbon. For additional information about allenes, including the nomenclature of dissymmetric allenes and models of enantiomeric 2,3-pentadiene Click Here.
.
By clicking on the above diagram, additional examples of axial chirality will be displayed. The substituted alkylidenecycloalkanes and spiro-bicyclic alkanes are analogous to the allene and cummulene systems if one considers the double bonds to be two-membered rings. Thus, depending on the number of such units linked together, the terminal substituents will either be orthogonal or coplanar. These configurations are relatively rigid. Converting one to the other requires breaking and making bonds.
Substituted biphenyls, on the other hand, exhibit a conformational enantiomorphism sometimes called
atropisomerism. The configurational stability of such isomers depends on the energy barrier to rotation about the single bond connecting the rings, and this in turn is proportional to the size of the ortho-substituents on each ring. The stereoisomerism of substituted biphenyls has been described elsewhere in this text, together with other examples.
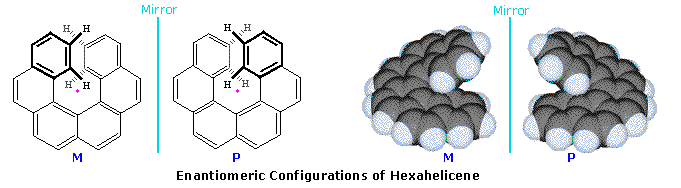
For a model of 2,6-dichlorospiro[3.3]heptane enantiomers . A similar axial dissymmetry is found in helical molecules, such as hexahelicene. Two views of the enantiomers of this interesting molecule are displayed below. When the configuration is viewed from above (or below) the helical turn, as shown by the structures on the left, its handedness may be established by the direction in which it turns. Imagine the helix is part of a screw, the axis of which is represented by the pink dot. If a clockwise turn of the screw would move it away from the viewer it is considered to have a
plus or
P configuration, also termed R
a or R
h by some. In contrast, if a counterclockwise turn moves the helix away it has a
minus or
M configuration, sometimes called S
a or S
h. Interactive models of enantiomeric hexahelicenes will be shown
by clicking on the diagram.
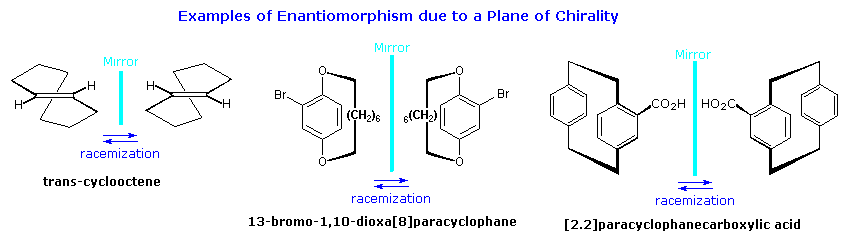
Molecules having a
plane of chirality are also known. Three examples are shown in the following diagram, and it should be noted that there is no asymmetric carbon present in any of them. In order to provide such chiral structures with a configurational prefix, a viewing rule has been established. First, the atom of highest priority (according to the CIP rules) that is directly bound to an atom in the chirality plane must be found. This atom, known as the pilot atom (
P), is the point from which the chiral plane is viewed. In the ansa compound, 13-bromo-1,10-dioxa[8]paracyclophane, the chiral plane is the aromatic ring. The pilot atom is the oxygen-bound methylene carbon atom closest to the bromine atom on the aromatic ring. Starting from the pilot atom, the next three consecutive atoms in the chirality plane are labelled
a,
b, and
c. In the case of branching options, the atom of highest CIP priority is selected. For the ansa compound, this leads to the aromatic carbon atom bound to bromine as
c. Finally, the absolute configuration is called
Sp or
M if the atom sequence
a–b–c, viewed from above the P–a bond, describes a counterclockwise arc. The configuration is termed
Rp or
P if this atom sequence describes a clockwise arc, as in its mirror image. The subscript
p denotes that the configuration is established relative to a plane of chirality.
By
clicking on the diagram the pilot atoms for each compound will be designated, and the
a–b–c sequence for the ansa compound will be displayed.
The chiral plane in
trans-cyclooctene is roughly the plane of the double bond, and in [2.2]paracyclophanecarboxylic acid it is roughly the plane of the aromatic ring bearing the carboxyl group. Due to strain, neither of these groups is truly coplanar, as may be seen in the three-dimensional models available below. Each model will display the pilot atom (there are two equivalent pilot atoms in
trans-cyclooctene) and the resulting configurational sequence of atoms.